Soil pH Explained: Not Necessarily Cool, but Extremely Important
Jul 13, 2022
 By: Robert Mullen, PhD, CCA, CPAg, VP Ag Technology
By: Robert Mullen, PhD, CCA, CPAg, VP Ag Technology In the realm of soil fertility, soil pH is not the coolest thing to discuss, but it just might be the most important.
Nutrient management explained
Here is an illustration a former academic advisor of mine used to describe nutrient management for non-legume crops:
Nutrient management is equated to vehicle maintenance. Nitrogen is the gas, phosphorus/potassium is like oil in the crankcase, and soil pH is like the air pressure of the tires. The length of the trip (yield potential) determines how much gas (nitrogen) is required for a given growing season. We should be able to complete a trip without adding oil (phosphorus/potassium) if the crankcase is full (soil testing reveals this). That leaves us with the air pressure, if the tires are flat (pH is way off for the crop being grown), no matter how much gas you add to the fuel tank or oil to the crankcase you are not going far. Like most analogies, this illustration does not adequately describe the reality, but it gives you the concept.
Why does pH matter so much?
Primarily because this simple soil chemical parameter dictates nutrient availability. The amount of nutrients available to a growing crop determines just how well it will yield. Most grasses prefer pH to be slightly acid to neutral (pH of about 6.0 to 7.0). Legumes prefer a slightly less acidic environment with desired pHs 6.8 or above.
So, what if your pH is too low?
You can decrease yield considerably, but it depends upon just how low your pH is. The United States Department of Agriculture’s Natural Resources Conservation Service (USDA-NRCS) sites a Soil Science Society of America publication that reports yield losses as much as 25-30% for corn when the soil pH is two units below the desired level. Other crops can experience even more yield loss.
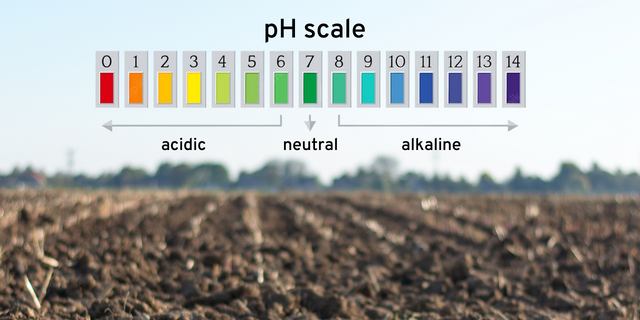
How do you correct the problem of your soil being too acidic?
One simple answer – lime. Soil pH tells you whether or not you need lime, and buffer pH tells you how much lime to add.

So, what if your pH is too high?
We do have some areas in Ohio that have soil pH levels higher than 7.0. High soil pH is typically more of an issue in the Western United States. High soil pH conditions can dramatically decrease micronutrient availability translating into yield loss. So, do not over-lime your soils. That can actually be detrimental.
Manage your pH
If you would like more information on managing soil pH and sourcing lime for your operation, reach out to your local Heritage agronomist.